 caseSensitive
caseSensitive compositional
compositional content
content copyright
copyright date
date- Values Differ
 description
description experimental
experimental hierarchyMeaning
hierarchyMeaning jurisdiction
jurisdiction
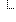 jurisdiction[0]
jurisdiction[0] name
name publisher
publisher purpose
purpose status
status title
title url
url version
version- Values Differ
 versionNeeded
versionNeeded| Error | CodeSystem.version | Values for version differ: '0.2.0' vs '0.2.1' |
| Information | CodeSystem.date | Values for date differ: '2023-10-24T08:16:05+00:00' vs '2023-10-31T17:40:25+00:00' |
| Name | Value | Comments | |
|---|---|---|---|
 caseSensitive caseSensitive | true | ||
 compositional compositional | |||
 content content | complete | ||
 copyright copyright | |||
 date date | 2023-10-24T08:16:05+00:00 | 2023-10-31T17:40:25+00:00 |
|
 description description | Définition des phases de l'essai utilisées dans la base de données ECLAIRE | ||
 experimental experimental | |||
 hierarchyMeaning hierarchyMeaning | |||
 jurisdiction jurisdiction | |||
 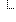 jurisdiction[0] jurisdiction[0] | urn:iso:std:iso:3166#FR | ||
 name name | EclaireStudyPhaseSourceCS | ||
 publisher publisher | ANS | ||
 purpose purpose | |||
 status status | draft | ||
 title title | Définition des phase de l'essai utilisés dans la base de données ECLAIRE | ||
 url url | https://interop.esante.gouv.fr/ig/fhir/eclaire/CodeSystem/eclaire-study-phase-source-code-system | ||
 version version | 0.2.0 | 0.2.1 |
|
 versionNeeded versionNeeded | |||
| Code | Display | Comments | |
|---|---|---|---|
 jarde-early jarde-early | Jardé phase précoce | ||
 phase-I-first-admin phase-I-first-admin | Human Pharmacology (Phase I) - First administration to humans | ||
 phase-I-bioequivalence phase-I-bioequivalence | Human Pharmacology (Phase I) - Bioequivalence Study | ||
 phase-I-other phase-I-other | Human Pharmacology (Phase I) - Other | ||
 phase-I-II-first-admin phase-I-II-first-admin | Phase I and Phase II (Integrated) - First administration to humans | ||
 phase-I-II-first-bioequivalence phase-I-II-first-bioequivalence | Phase I and Phase II (Integrated) - Bioequivalence Study | ||
 phase-I-II-other phase-I-II-other | Phase I and Phase II (Integrated) - Other | ||
 phase-II phase-II | Therapeutic exploratory (Phase II) | ||
 phase-II-III phase-II-III | Phase II and Phase III (Integrated) | ||
 phase-III phase-III | Therapeutic confirmatory (Phase III) | ||
 phase-IV phase-IV | Therapeutic use (Phase IV) | ||
 phase-III-IV phase-III-IV | Phase III and phase IV (Integrated) | ||